Water Confinement on Polymer Coatings Dictates Proton−ElectronTransfer on Metal-Catalyzed Hydrogenation of Nitrite, was published in the JACS Au Journal by ACS Publications
Prof. Jimmy Faria Albanese tells us this:
Few years ago, we asked these questions: What would be the effect of stimulus-responsive polymers on the solvation layer near the active site and how would these affect change the Proton Electron Transfer mechanism? The idea of using stimulus-responsive polymers is that you could modulate the fate of the reactions occurring on the catalyst in a similar fashion as living-organisms regulate their temperature. That is a self-regulating or homeostatic behaviour.
To address these questions we studied the reduction of nitrite ions in water over palladium, which is a reaction extremely relevant for water treatment.
Here, we found that the polymer in its swollen state interacts with the water molecules near the active site via hydrogen bonding. The water near the active site becomes less mobile, which in turn helps to reduce the enthalpy of activation for the transition state of the PET step. This stabilisation comes at the expense of reducing the degrees of freedom, which in turn reduces the entropy of activation. We showed that this stabilisation of the transition state does not come from a stronger binding of the kinetically relevant species prior the transition state. It is instead a change in the energetics of the TS itself. The beauty of this concept, in my opinion, is that as the polymer collapses on itself these effects vanish and the kinetics resembled that of the uncoated catalyst. Like the polymer was never there!
We still have many unanswered questions. For example, we know that there are spontaneous electric fields formed between the metal and the water when conducting hydrogenation reactions, which ultimately dictate the rate of the reaction. In this system, however, we do not know exactly how these stimulus-responsive polymer alter the dielectrics of the media near the active site and therefore the strength of the electric fields. We also do not know what is the exact manner in which the TS is stabilised. We know that the water is less mobile and that the apparent barriers are lower, but we still have not done full DFT calculations of the energy barriers in the presence of the polymer. We also do not know exactly how these polymers interact with the metal surface. Do they change the work function of the metal or is this surface unaffected?
Having said that, I wanted to thank Prof. Bin Wang and Yu Yan for their tireless efforts on the computational side. This work was only possible thanks to this collaboration!
A special thank to Pengcheng Huang who dedicated endless hours on the experimental work on the reaction kinetics and materials synthesis, and Ricardo Martinho for the fantastic work on the NMR. Also, many thanks to my colleague Prof. Leon Lefferts who helped us in sharpening the story and challenge our ideas.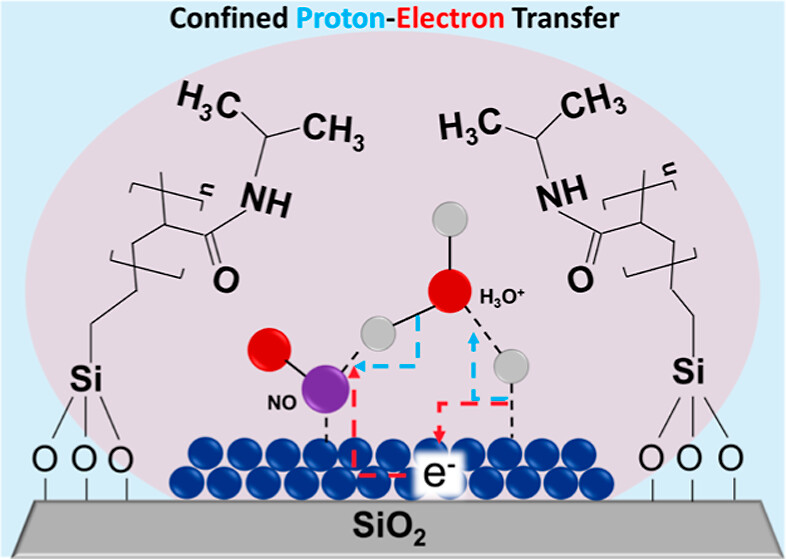
hashtag#catalysis hashtag#smart_materials hashtag#nitrogen_removal hashtag#water_treatment




